


Additional drug – drug interactions are noted for awareness.
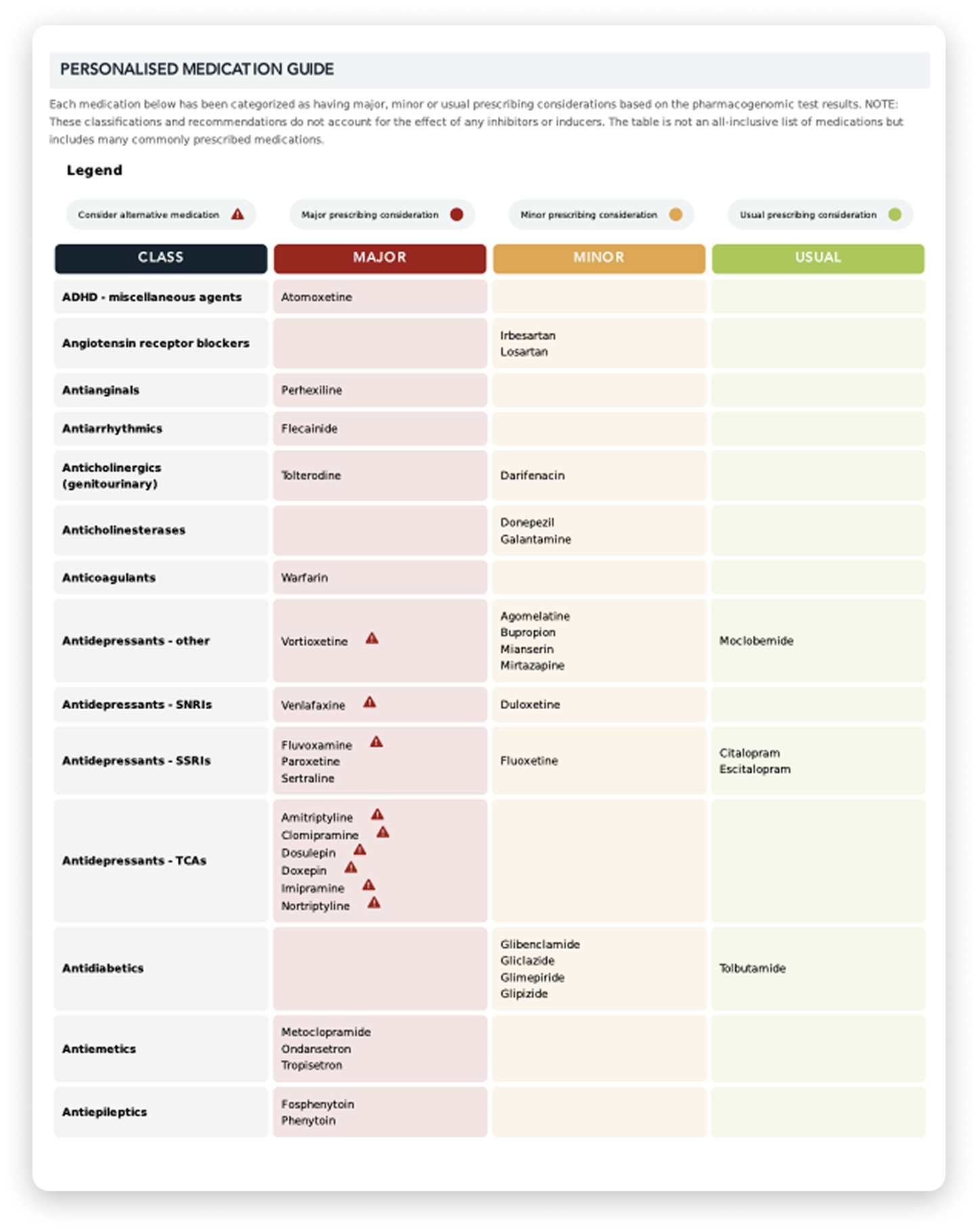


In my laboratory work with clinics, I’ve seen how pharmacogenomics testing can give patients extra answers.
One woman with depression tried several antidepressants, but her symptoms kept getting worse and she ended up in hospital.
Pharmacogenomics testing suggested those medicines may not have been the right fit, so we adjusted her treatment to something that appeared more suitable. Over time, her symptoms improved.
I’ve also seen patients whose main challenge was side effects. One woman’s mood improved on medication, but the nausea and weight loss made her want to stop.
Pharmacogenomics testing indicated her medicine may break down more slowly, which could explain why she felt unwell. By lowering the dose, she was able to keep taking it with fewer side effects.
These experiences show how PGx testing can give doctors useful information to help guide treatment choices

Test results for the PGx Multi are downloaded into your practice management software in the usual manner.
There is two reports, a summary report and a detailed pdf report. The latter pdf report contains additional information on prescribing for the patient, based on international guidelines.
If you are not receiving this additional pdf report please let us know on 1800 822 999.
The PGx Multi is a non-Medicare rebateable test. Please ask your patients to pay via the shopping cart on this website or or call 1800 822 999 before having their bloods collected.
Simply request PGx Multi on a standard request form. It is important to list all medications the patient is currently taking, so an accurate report can be returned.
(1) Van Driest SL, ShiY, Bowton EA, Schildcrout JS, Peterson JF, Pulley J, Denny JC, Roden DM.Clinically actionable genotypes among 10,000 patients with preemptive pharmacogenomic testing. Clin Pharmacol Ther. 2014 Apr;95(4):423-31. doi:10.1038/clpt.2013.229. Epub 2013 Nov 19. PMID: 24253661; PMCID: PMC3961508.
(2) KomagamineJ. Prevalence of urgent hospitalizations caused by adverse drug reactions: across-sectional study. Sci Rep. 2024 Mar 13;14(1):6058. doi:10.1038/s41598-024-56855-z. PMID: 38480855; PMCID: PMC10937656.
(3) ChengY, Liu H, Yuan R, Yuan K, Yu S. Effectiveness of pharmacogenomics on theresponse and remission of treatment-resistant depression: a meta-analysis ofrandomised controlled trials. General Psychiatry. 2023;36:e101050.
(4) Bergfeld IO, Mantione M, Figee M,Schuurman PR, Lok A, Denys D. Treatment-resistant depression and suicidality. JAffect Disord. 2018 Aug 1;235:362-367. doi: 10.1016/j.jad.2018.04.016. Epub2018 Apr 3. PMID: 29665520.
(5) Rush AJ, Trivedi MH, Wisniewski SR,Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, LebowitzBD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acuteand longer-term outcomes in depressed outpatients requiring one or severaltreatment steps: a STAR*D report. Am J Psychiatry. 2006 Nov;163(11):1905-17.doi: 10.1176/ajp.2006.163.11.1905. PMID: 17074942.
(6) ChengY, Liu H, Yuan R, Yuan K, Yu S. Effectiveness of pharmacogenomics on theresponse and remission of treatment-resistant depression: a meta-analysis ofrandomised controlled trials. General Psychiatry. 2023;36:e101050.
(7) Bergfeld IO, Mantione M, Figee M,Schuurman PR, Lok A, Denys D. Treatment-resistant depression and suicidality. JAffect Disord. 2018 Aug 1;235:362-367. doi: 10.1016/j.jad.2018.04.016. Epub2018 Apr 3. PMID: 29665520.
(8) Rush AJ, Trivedi MH, Wisniewski SR,Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, LebowitzBD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acuteand longer-term outcomes in depressed outpatients requiring one or severaltreatment steps: a STAR*D report. Am J Psychiatry. 2006 Nov;163(11):1905-17.doi: 10.1176/ajp.2006.163.11.1905. PMID: 17074942.
(9) https://www.rcpa.edu.au/Library/Practising-Pathology/Pharmacogenomic-Indications-in-Australia
(10) KomagamineJ. Prevalence of urgent hospitalizations caused by adverse drug reactions: across-sectional study. Sci Rep. 2024 Mar 13;14(1):6058. doi:10.1038/s41598-024-56855-z. PMID: 38480855; PMCID: PMC10937656.
(11) Sugarman, E.A.,Cullors, A., Centeno, J. et al. Contribution ofPharmacogenetic Testing to Modeled Medication Change Recommendations in aLong-Term Care Population with Polypharmacy. Drugs Aging 33,929–936 (2016).
(12) https://www.rcpa.edu.au/Library/Practising-Pathology/Pharmacogenomic-Indications-in-Australia
(13) Van Driest SL, ShiY, Bowton EA, Schildcrout JS, Peterson JF, Pulley J, Denny JC, Roden DM.Clinically actionable genotypes among 10,000 patients with preemptivepharmacogenomic testing. Clin Pharmacol Ther. 2014 Apr;95(4):423-31. doi:10.1038/clpt.2013.229. Epub 2013 Nov 19. PMID: 24253661; PMCID: PMC3961508.
(14) Kimpton JE, Carey IM, Threapleton CJD, et al. Longitudinalexposure of English primary care patients to pharmacogenomic drugs: An analysisto inform design of pre-emptive pharmacogenomic testing. Br JClin Pharmacol. 2019; 85: 2734–2746.
(15) ChengY, Liu H, Yuan R, Yuan K, Yu S. Effectiveness of pharmacogenomics on theresponse and remission of treatment-resistant depression: a meta-analysis ofrandomised controlled trials. General Psychiatry. 2023;36:e101050.
(16) Bergfeld IO, Mantione M, Figee M,Schuurman PR, Lok A, Denys D. Treatment-resistant depression and suicidality. JAffect Disord. 2018 Aug 1;235:362-367. doi: 10.1016/j.jad.2018.04.016. Epub2018 Apr 3. PMID: 29665520.
(17) Rush AJ, Trivedi MH, Wisniewski SR,Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, LebowitzBD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acuteand longer-term outcomes in depressed outpatients requiring one or severaltreatment steps: a STAR*D report. Am J Psychiatry. 2006 Nov;163(11):1905-17.doi: 10.1176/ajp.2006.163.11.1905. PMID: 17074942.
(18) ChengY, Liu H, Yuan R, Yuan K, Yu S. Effectiveness of pharmacogenomics on theresponse and remission of treatment-resistant depression: a meta-analysis ofrandomised controlled trials. General Psychiatry. 2023;36:e101050.
(19) Bergfeld IO, Mantione M, Figee M,Schuurman PR, Lok A, Denys D. Treatment-resistant depression and suicidality. JAffect Disord. 2018 Aug 1;235:362-367. doi: 10.1016/j.jad.2018.04.016. Epub2018 Apr 3. PMID: 29665520.
(20) Rush AJ, Trivedi MH, Wisniewski SR,Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, LebowitzBD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acuteand longer-term outcomes in depressed outpatients requiring one or severaltreatment steps: a STAR*D report. Am J Psychiatry. 2006 Nov;163(11):1905-17.doi: 10.1176/ajp.2006.163.11.1905. PMID: 17074942.
(21) KomagamineJ. Prevalence of urgent hospitalizations caused by adverse drug reactions: across-sectional study. Sci Rep. 2024 Mar 13;14(1):6058. doi:10.1038/s41598-024-56855-z. PMID: 38480855; PMCID: PMC10937656.
(22) Bousman CA, Arandjelovic K, Mancuso SG,Eyre HA, Dunlop BW. Pharmacogenetic tests and depressive symptom remission: ameta-analysis of randomized controlled trials. Pharmacogenomics. 2019Jan;20(1):37-47. doi: 10.2217/pgs-2018-0142. Epub 2018 Dec 6. PMID: 30520364.
(23) Sugarman, E.A.,Cullors, A., Centeno, J. et al. Contribution ofPharmacogenetic Testing to Modeled Medication Change Recommendations in aLong-Term Care Population with Polypharmacy. Drugs Aging 33,929–936 (2016).
(24) MacielA, Cullors A, Lukowiak AA, Garces J. Estimating cost savings of pharmacogenetictesting for depression in real-world clinical settings. Neuropsychiatr DisTreat. 2018 Jan 8;14:225-230. doi: 10.2147/NDT.S145046. PMID: 29386895; PMCID:PMC5764291.
(25) MacielA, Cullors A, Lukowiak AA, Garces J. Estimating cost savings of pharmacogenetictesting for depression in real-world clinical settings. Neuropsychiatr DisTreat. 2018 Jan 8;14:225-230. doi: 10.2147/NDT.S145046. PMID: 29386895; PMCID:PMC5764291.
(26) https://www.rcpa.edu.au/Library/Practising-Pathology/Pharmacogenomic-Indications-in-Australia
(27) MacielA, Cullors A, Lukowiak AA, Garces J. Estimating cost savings of pharmacogenetictesting for depression in real-world clinical settings. Neuropsychiatr DisTreat. 2018 Jan 8;14:225-230. doi: 10.2147/NDT.S145046. PMID: 29386895; PMCID:PMC5764291.
1. I have experienced unwanted side effects from medications.