




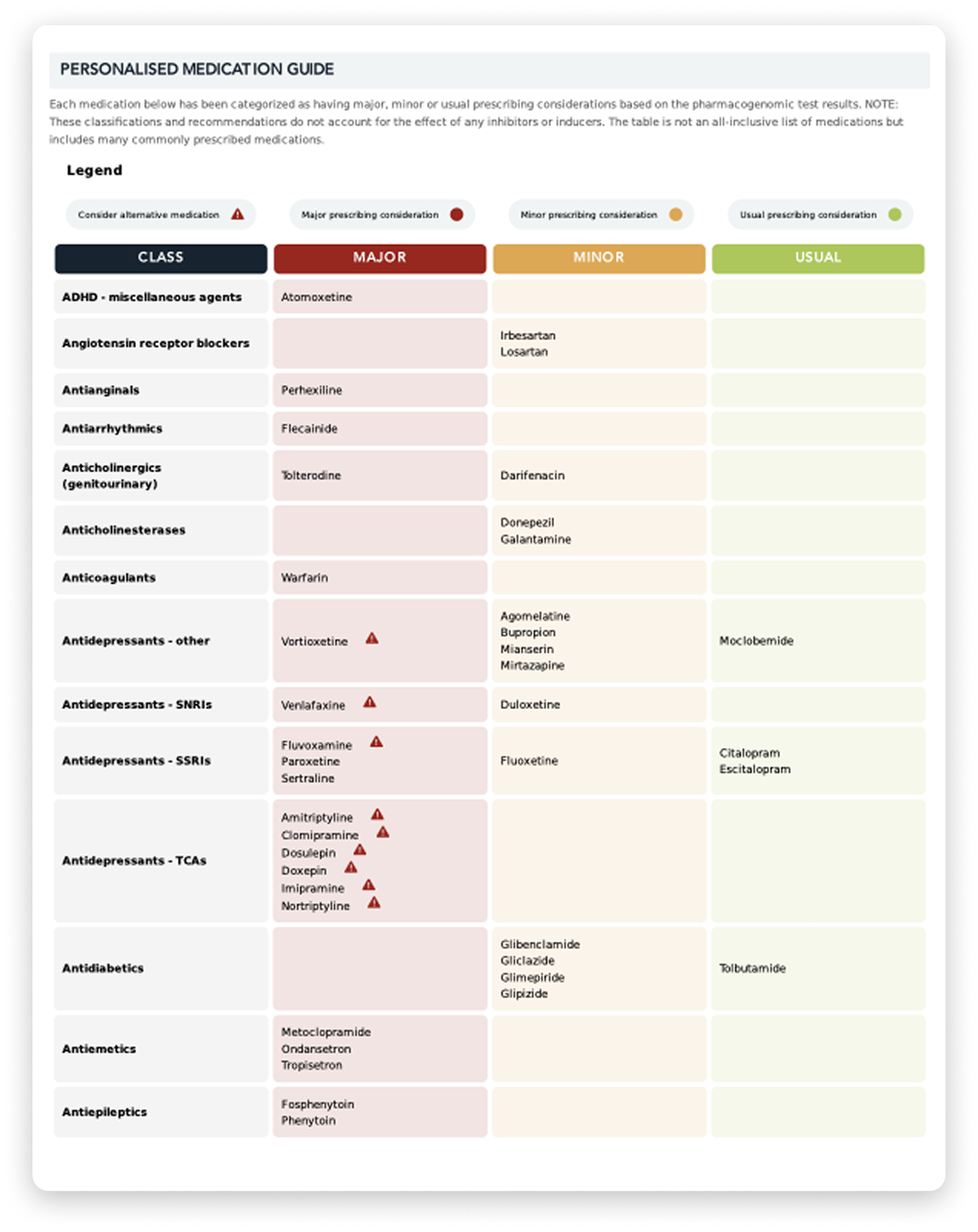


In my laboratory work with clinics, I’ve seen how pharmacogenomics testing can give patients extra answers.
One woman with depression tried several antidepressants, but her symptoms kept getting worse and she ended up in hospital.
Pharmacogenomics testing suggested those medicines may not have been the right fit, so we adjusted her treatment to something that appeared more suitable. Over time, her symptoms improved.
I’ve also seen patients whose main challenge was side effects. One woman’s mood improved on medication, but the nausea and weight loss made her want to stop.
Pharmacogenomics testing indicated her medicine may break down more slowly, which could explain why she felt unwell. By lowering the dose, she was able to keep taking it with fewer side effects.
These experiences show how pharmacogenomics testing can give doctors useful information to help guide treatment choices

You will need to contact us on 1800 822 999 or at info@genomicdiagnositics.com.au We will then organise for you to attend one of our Healius Collection Centres for a recollection of your sample.
The results of your pharmacogenomics test results (report) will be sent to your doctor approximately 3-4 weeks from the date the test swab is sent back to us in the post. You will need to make an appointment to see your doctor to discuss the results.
Yes, you can select and pay for Express Post, which delivers in approximately - 5 days. There is an added cost of $15 for this service.
Australia Post standard small parcel service normally delivers within 6 - 10 business days.
Yes, you will need to secure a TRF from your doctor, and send us a copy via the link (www.genomicdiagnostics.com.au/trf) before we can send the Home Testing Kit to you.
1. I have experienced unwanted side effects from medications.