Familial Cancer Testing
Familial Cancer Testing is used to determine if a patient has a predisposition to
cancer due to genetic variants that are inherited (passed down through the
family).
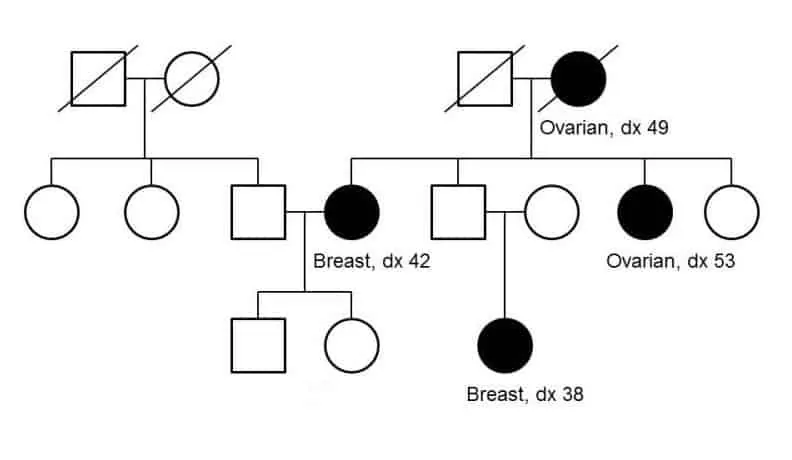
Well-known familial cancer syndromes include hereditary breast and ovarian cancer (HBOC) syndrome and Lynch syndrome.
Some individuals with a family history of cancer may be at increased risk through the inheritance of genetic variants in particular genes associated with cancer predisposition. Testing for these genetic variants is important as it can make a diagnosis or determine risk of cancer, and may also offer insight regarding appropriate prevention, surveillance, or treatment strategies.
For HBOC, a number of high and moderate risk genes that have been demonstrated to increase risk of breast and/or ovarian cancer can be tested. Testing of genes associated with Lynch and familial adenomatous polyposis (FAP) syndromes can also be important in the setting of inherited colorectal cancer.
Testing to detect genetic variants in the BRCA1 and BRCA2 genes is also used to determine eligibility for olaparib therapy in some patients with ovarian, breast or prostate cancer.
Genomic Diagnostics offer two pathways by which patients may be tested for hereditary breast and ovarian cancer variants. All testing for colorectal cancer syndromes is only accepted via the specialist pathway.
Leading the way to improved health
results in the shortest turnaround time.